Master The Art Of Counting Atoms: How To Count Atoms
Counting atoms may seem like a daunting task, but fear not! The key to mastering this fundamental skill lies in understanding the basic principles of chemistry. By learning how to count atoms, you’ll be able to navigate the complexities of chemical equations with ease and precision. Ready to unlock the secrets of the atomic world? Let’s dive in and discover the simple yet powerful techniques for counting atoms.
Exploring the World of Atoms: A Beginner’s Guide to Counting Atoms
How to Count Atoms
Welcome, young scientists! Today, we are going to embark on an exciting journey into the realm of atoms. Have you ever wondered
how many atoms are in a molecule? Don’t worry; we will unravel this mystery together. In this guide, we will learn the basics
of counting atoms, understand the rules that govern this process, and even try some fun exercises to test our newfound knowledge.
Let’s dive in!
What Are Atoms?
Before we learn how to count atoms, let’s understand what atoms are. Atoms are the building blocks of everything around us.
They are tiny particles that make up all matter in the universe. Imagine atoms as the LEGO bricks of nature, joining together
to form molecules and substances.
Each atom is made up of even smaller particles called protons, neutrons, and electrons. Protons have a positive charge, neutrons
carry no charge, and electrons have a negative charge. These subatomic particles determine the properties of different atoms.
Atomic Number and Atomic Mass
Now, let’s introduce two crucial concepts in the world of atoms: atomic number and atomic mass. The atomic number of an atom
tells us the number of protons in its nucleus. For example, hydrogen has an atomic number of 1, which means it has one proton.
On the other hand, the atomic mass of an atom is the sum of its protons and neutrons. This value gives us the total mass of the
atom. For oxygen, which has 8 protons and 8 neutrons, the atomic mass is 16.
Counting Atoms in a Molecule
Now, let’s move on to the exciting part: counting atoms in a molecule. A molecule is a group of atoms bonded together. To count
atoms in a molecule, we need to look at the chemical formula. A chemical formula shows the types and numbers of atoms present in
a compound.
Let’s take a simple molecule like water, which has the chemical formula H2O. This formula tells us that water consists of 2 hydrogen
atoms and 1 oxygen atom. The numbers next to the elements indicate how many atoms of each element are present in the molecule.
Rules for Counting Atoms
There are some rules we need to follow when counting atoms in a molecule:
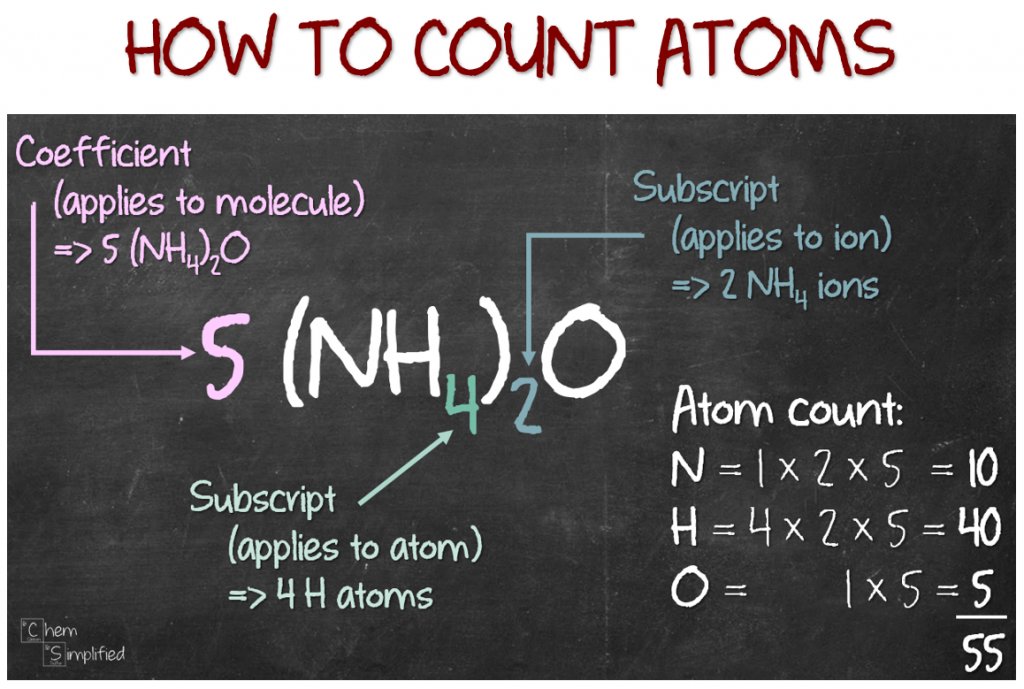
- Subscripts: The small numbers written to the right of the element symbol indicate the number of atoms of that
element. For example, in CO2, there are 2 oxygen atoms. - Parentheses: Parentheses are used to group atoms together. The number outside the parentheses applies to all
atoms inside. For example, (NH4)2SO4 contains 2 ammonium groups and 1 sulfate group. - Coefficients: Coefficients outside the chemical formula apply to all the atoms inside. If there is no subscript
after an element, it means there is one atom of that element.
Practice Makes Perfect
Let’s put our atom-counting skills to the test with some practice exercises:
Exercise 1: Counting Atoms in Carbon Dioxide (CO2)
How many oxygen atoms are in one molecule of carbon dioxide?
Exercise 2: Counting Atoms in Ammonium Bicarbonate ([NH4]HCO3)
How many hydrogen atoms are present in one molecule of ammonium bicarbonate?
Try solving these exercises on your own and compare your answers with a friend or a teacher. Remember, practice leads to perfection!
Congratulations, young scientists! You have now learned the basics of counting atoms in molecules. Remember, atoms are the fundamental
units of matter, and understanding how they combine to form compounds is the key to unlocking the secrets of chemistry.
Keep exploring the fascinating world of atoms, and who knows, you might make groundbreaking discoveries one day. Happy atom counting!
Counting Atoms
Frequently Asked Questions
How do you count atoms in a chemical formula?
To count atoms in a chemical formula, first identify the elements present. Next, determine the subscript next to each element, indicating the number of atoms of that element in the compound. Multiply the subscript by the number of atoms if there is more than one of that element present. Finally, add up the total number of atoms of each element to get the overall count.
Why is it important to accurately count atoms in a compound?
Accurately counting atoms in a compound is crucial for understanding the chemical composition and properties of the substance. It helps in predicting chemical reactions, determining stoichiometry, and balancing chemical equations, which are essential in various fields such as chemistry, biology, and material science.
Can you explain how to count atoms when there are parentheses in the chemical formula?
When dealing with parentheses in a chemical formula, multiply the subscript outside the parentheses by the subscripts inside the parentheses. This distributes the subscript to all the elements enclosed in the parentheses. Then, proceed with counting atoms of each element as usual by considering the multiplied subscripts.
Final Thoughts
In conclusion, understanding how to count atoms is essential for mastering chemistry. By following simple steps like identifying the elements present and using the mole concept, anyone can accurately determine the number of atoms in a compound. Practice and patience are key to improving atom counting skills. Happy counting!